what type of bonds are shown in this diagram
The first two equations illustrate the initiation process and the last two equations are examples of chain propagation. Enzyme structures unfold when heated or exposed to chemical denaturants.
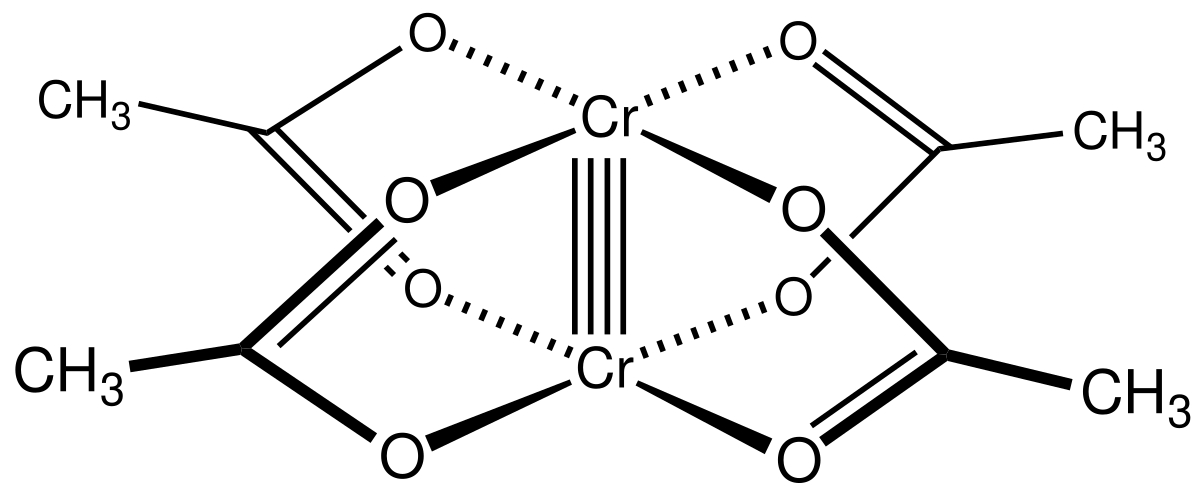
Quadruple Bond Wikipedia
Energy Band Diagram of p-Type Semiconductor.
. The triple point and the critical point are shown as red dots. Animated diagram of a half-wave dipole antenna receiving a radio wave. Pi bonds are usually weaker than sigma bondsThe C-C double bond composed of one sigma and one pi bond has a bond energy less than twice that of a C-C single bond indicating that the stability added by the pi bond is less than the stability of a sigma bond.
A large number of holes or vacant space in the covalent bond is created in the crystal with the addition of the trivalent impurity. The solid green line shows the usual shape of the liquidsolid phase line. Most steroid users are not athletes.
Energy Diagram of n-Type Semiconductor. Some circulates in an open cavity. The dotted green line shows the anomalous behavior of water when the pressure increases.
Then you describe the specific details of the paper you need. In the United States between 1 million and 3 million people 1 of the population are thought to have used AAS. Every solid liquid gas and plasma is composed of neutral or ionized atoms.
A pure gas may be made up of individual atoms eg. Oxygen or compound molecules made from a variety of atoms eg. A typical phase diagram for a single-component material exhibiting solid liquid and gaseous phases.
Atoms are extremely small typically around 100 picometers across. Browse our listings to find jobs in Germany for expats including jobs for English speakers or those in your native language. The energy band diagram of a p-type Semiconductor is shown below.
Insects from Latin insectum are pancrustacean hexapod invertebrates of the class InsectaThey are the largest group within the arthropod phylumInsects have a chitinous exoskeleton a three-part body head thorax and abdomen three pairs of jointed legs compound eyes and one pair of antennaeTheir blood is not totally contained in vessels. For many molecules the sharing of electrons allows each atom to attain the. A fossil fuel petroleum is formed when large quantities of dead organisms.
From the perspective of quantum mechanics this bonds weakness is explained by significantly less overlap between. The Energy level diagram of the n-type semiconductor is shown in the figure below. Petroleum also known as crude oil or simply oil is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons and is found in geological formationsThe name petroleum covers both naturally occurring unprocessed crude oil and petroleum products that consist of refined crude oil.
About a purchase you have made. The electric field E green arrows of the incoming wave pushes the electrons in the rods back and forth charging the ends alternately positive and negative. Your academic level paper type and format the number of pages and sources discipline and deadline.
In chemistry the empirical formula of a chemical is a simple expression of the relative number of each type of atom or ratio of the elements in the compound. One example of this radical polymerization is the conversion of styrene to polystyrene shown in the following diagram. Insulin is also used along with glucose to treat.
As a medication insulin is any pharmaceutical preparation of the protein hormone insulin that is used to treat high blood glucose. Empirical formulae are the standard for ionic compounds such as CaCl 2 and for macromolecules such as SiO 2An empirical formula makes no reference to isomerism structure or absolute number of atoms. We provide assignment help in over 80 subjects.
Gas is one of the four fundamental states of matter the others being solid liquid and plasma. Such conditions include type 1 diabetes type 2 diabetes gestational diabetes and complications of diabetes such as diabetic ketoacidosis and hyperosmolar hyperglycemic states. Order status placement and cancellation.
Ribonucleic acid RNA is a polymeric molecule essential in various biological roles in coding decoding regulation and expression of genesRNA and deoxyribonucleic acid are nucleic acidsAlong with lipids proteins and carbohydrates nucleic acids constitute one of the four major macromolecules essential for all known forms of lifeLike DNA RNA is assembled as a chain of. We are a leading online assignment help service provider. An atom is the smallest unit of ordinary matter that forms a chemical element.
Add the topic write or paste the instructions and attach files to be used if you have them. The carbon atom labeled C 1 appears to have only one bond so there must also be three hydrogens bonded to it in order to make its total number of bonds four. All your academic needs will be taken care of as early as you need them.
By using small amounts of initiators a wide variety of monomers can be polymerized. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. Get 247 customer support help when you place a homework help service order with us.
When a photon is absorbed its energy is given to an electron in the crystal lattice. You fill in the order form with your basic requirements for a paper. They are so small that accurately predicting their behavior using classical physics as if they were tennis balls for example is not possible due to quantum effects.
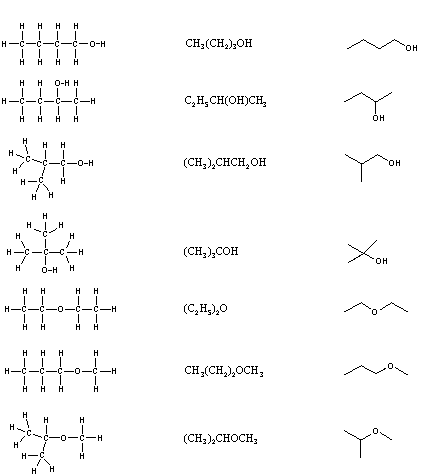
For example the skeletal formula of hexane top is shown below. The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell giving it the same electronic configuration as a noble gasThe rule is especially applicable to carbon nitrogen oxygen and the halogens. Carbon dioxideA gas mixture such as air contains a variety of pure gases.
In the fourth covalent bonds. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. The sanctions can be either comprehensive or selective using the blocking of assets and trade restrictions to accomplish foreign policy and national security goals.
Where is OFACs country list. Although more generally the rule is applicable for the s-block and p. A small or minute quantity of free electrons is also available in the.
Usually this electron is in the valence bandThe energy given to the electron by the photon excites it into the conduction band where it is free to move around within the semiconductor. A large number of free electrons are available in the conduction band because of the addition of the Pentavalent impurity. Implicit carbon and hydrogen atoms.
Studies in the United States have shown that AAS users tend to be mostly middle-class men with a median age of about 25 who are noncompetitive bodybuilders and non-athletes and use the drugs for cosmetic purposes. These electrons are free electrons which did not fit in the covalent bonds of the crystal. Although structure determines function a novel enzymatic activity cannot yet be predicted from structure alone.
The antenna consists of two metal rods connected to a receiver R. The carbon atom labelled C 3 has two bonds to other carbons and is therefore bonded to two hydrogen atoms as well. Enzymes are generally globular proteins acting alone or in larger complexesThe sequence of the amino acids specifies the structure which in turn determines the catalytic activity of the enzyme.
You can request for any type of assignment help from our highly qualified professional writers. OFAC administers a number of different sanctions programs. A noble gas like neon elemental molecules made from one type of atom eg.
The network of covalent bonds that the electron was previously a part of now has one fewer electron.
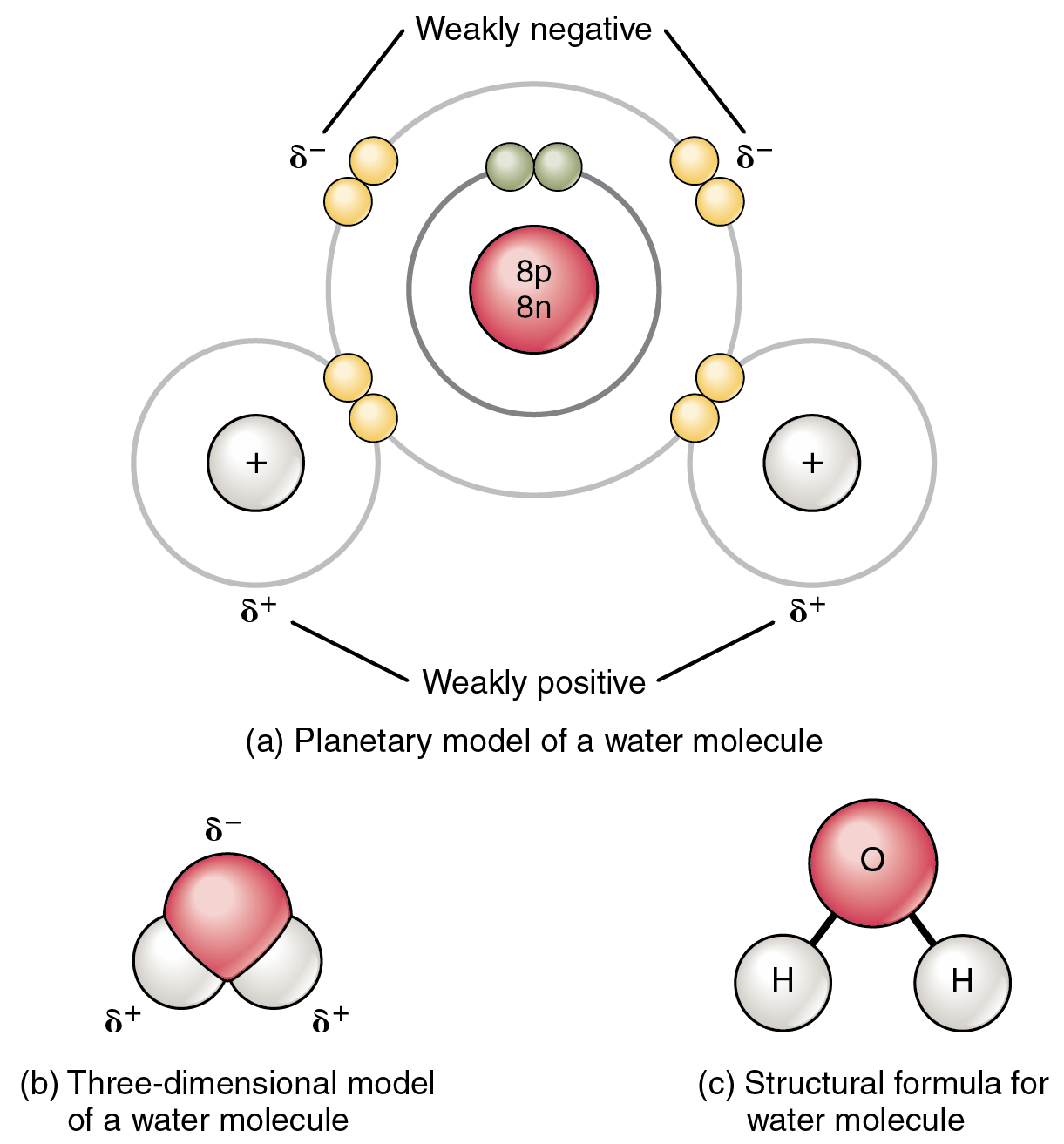
2 2 Chemical Bonds Anatomy Physiology
Covalent Bonding
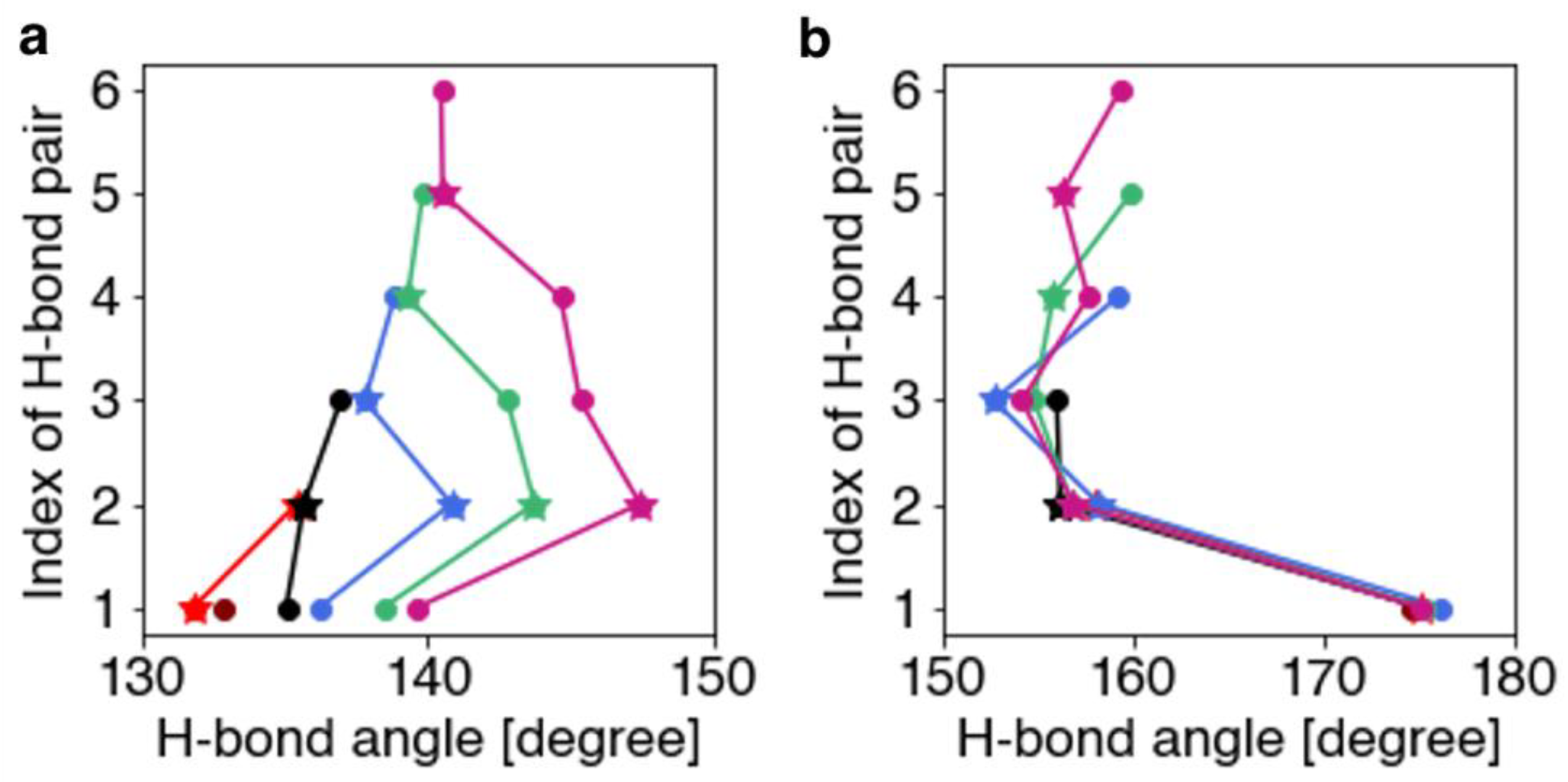
Ijms Free Full Text Depolarizing Effects In Hydrogen Bond Energy In 310 Helices Revealed By Quantum Chemical Analysis Html
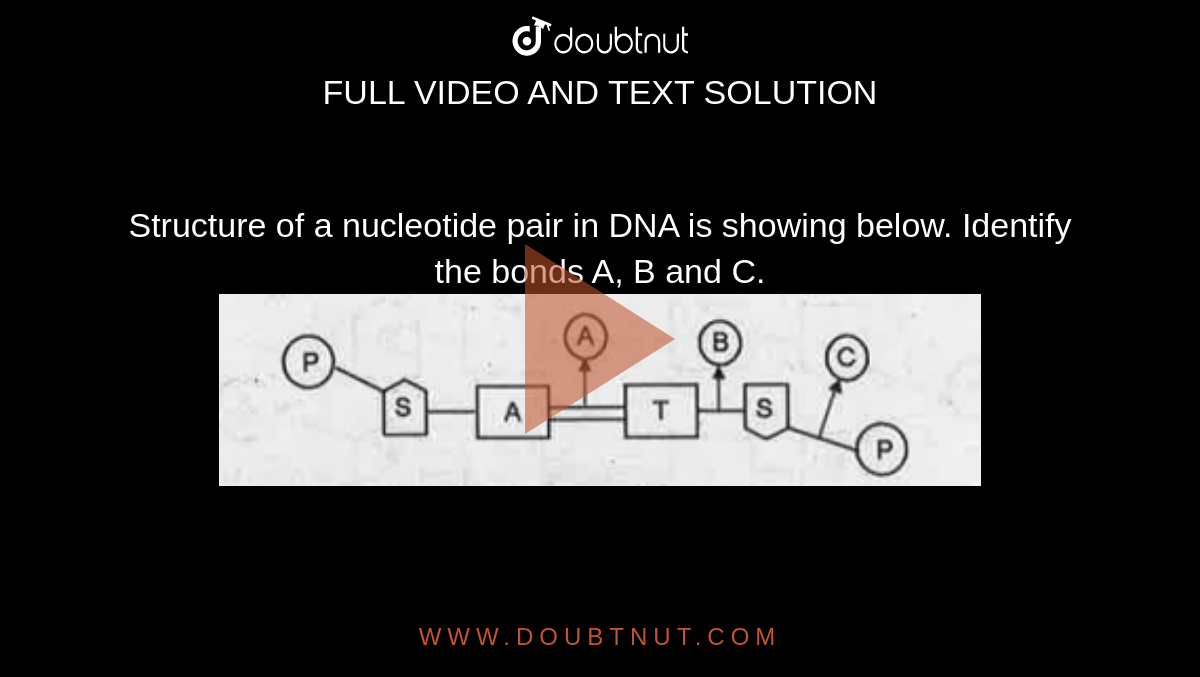
A N Glycosidic Bonding B Phosphodiester Bonding C Hydrogen Bonding

Carbon Bonds Overview List How Many Bonds Does Carbon Make Video Lesson Transcript Study Com
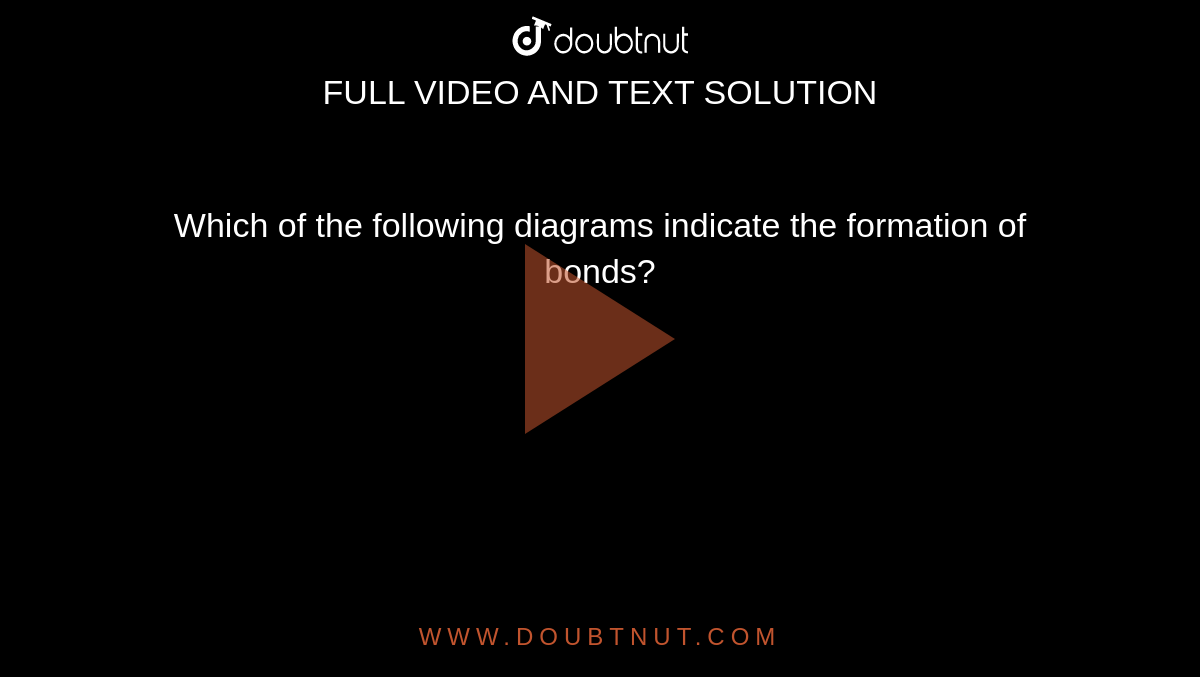
X Y Glycosidic Bond Hydrogen Bond

Intramolecular Covalent Bonds In Gram Positive Bacterial Surface Proteins Ma Chembiochem Wiley Online Library

Molecule Definition Examples Structures Facts Britannica

6 4 Ionic Bonding Chemical Bonding Siyavula

Chemical Bonds Anatomy And Physiology I

Hillis2e Ch02
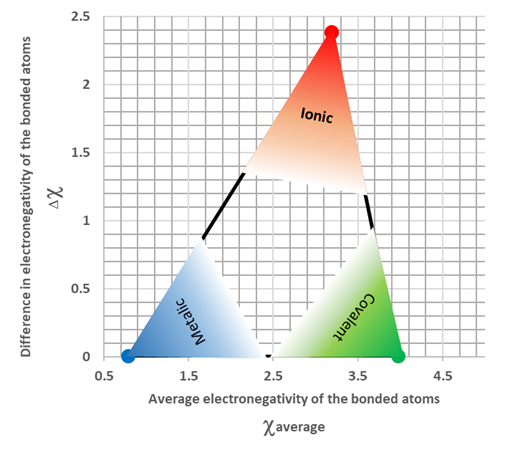
3 9 Intramolecular Forces And Intermolecular Forces Chemistry Libretexts

Chemical Bonds Principles Of Biology

Carbon Bonds Overview List How Many Bonds Does Carbon Make Video Lesson Transcript Study Com

Ionic Bond Definition Properties Examples Facts Britannica

A Schematic Diagram Shows The Types Of Hydrogen Bonds In Water Download Scientific Diagram
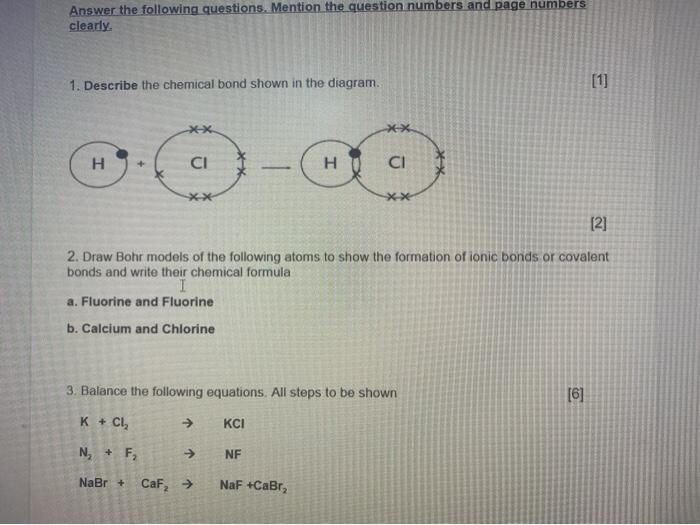
Solved Answer The Following Questions Mention The Question Chegg Com